Paul Griffin, The University of Queensland
As the COVID virus continues to evolve, so does our vaccine response. From 11 December, Australians will have access to new vaccines that offer better protection.
These ‘monovalent’ booster vaccines are expected to be a better match for currently circulating strains of SARS-CoV-2, the virus that causes COVID.
Pfizer’s monovalent vaccine will be available to eligible people aged five years and older. The Moderna monovalent vaccine can be used for those aged 12 years and older.
Who is eligible for these new boosters? How do they differ from earlier ones? Do they work? Are they safe?
Who’s eligible for the new boosters?
The federal government has accepted the Australian Technical Advisory Group (ATAGI) recommendation to use the new vaccines, after Australia’s regulator approved their use last month. However, vaccine eligibility has remained the same since September.
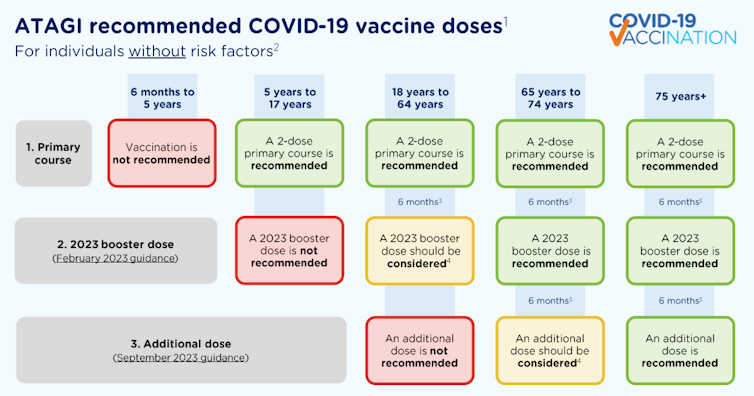
ATAGI recommends Australians aged over 75 get vaccinated if it has been six months or more since their last dose.
People aged 65 to 74 are recommended to have a 2023 booster if they haven’t already had one.
Health.gov.au
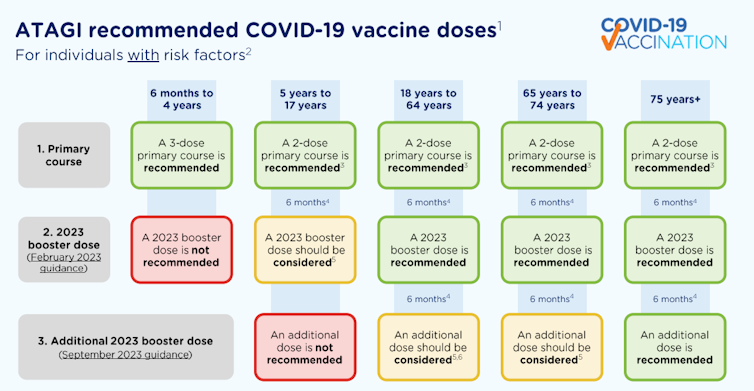
Adults aged 18 to 64 with underlying risk factors that increase their risk of severe COVID are also recommended to have a 2023 booster if they haven’t had one yet. And if they’ve already had a 2023 booster, they can consider an additional dose.
Health.gov.au
For adults aged 18 to 64 without underlying risk factors who have already received a 2023 booster, an additional dose isn’t recommended. But if you’re aged 18 to 64 and haven’t had a booster in 2023, you can consider an additional dose.
Additional doses aren’t recommended for children without underlying conditions that increase their risk of severe COVID. A primary course is not recommended for children aged six months to five years without additional risk factors.
Monovalent, bivalent? What’s the difference?
From monovalent
The initial COVID vaccines were ‘monovalent’. They had one target – the original viral strain.
But as the virus mutated, we assigned new letters of the Greek alphabet to each variant. This brings us to Omicron. With this significant change, we saw ‘immune evasion’. The virus had changed so much the original vaccines didn’t provide sufficient immunity.
To bivalent
So vaccines were updated to target an early Omicron subvariant, BA.1, plus the original ancestral strain. With two targets, these were the first of the ‘bivalent’ vaccines, which were approved in Australia in 2022.
Omicron continued to evolve, leading to more ‘immune escape’, contributing to repeated waves of transmission.
The vaccines were updated again in early 2023. These newer bivalent vaccines target two strains – the ancestral strain plus the subvariants BA.4 and BA.5.
Back to monovalent
Further changes in the virus have meant our boosters needed to be updated again. This takes us to the recent announcement.
This time the booster targets another subvariant of Omicron known as XBB.1.5 (sometimes known as Kraken).
This vaccine is monovalent once more, meaning it has only one target. The target against the original viral strain has been removed.
According to advice given to the World Health Organization in May, this is largely because immunity to this original strain is no longer required (it’s no longer infecting humans). Raising immunity to the original strain may also hamper the immune response to the newer component, but we’re not sure if this is occurring or how important this is.
The United States approved XBB.1.5-specific vaccines from Pfizer and Moderna in mid-September. These updated vaccines have also been approved in places including Europe, Canada, Japan and Singapore.
In Australia, the Therapeutic Goods Administration (TGA) approved
them in October.
Do these newer vaccines work?
Evidence for the efficacy of these new monovalent vaccines comes from the results of research Pfizer and Moderna submitted to the TGA.
Evidence also comes from a preprint (preliminary research available online that has yet to be independently reviewed) and an update Pfizer presented to the US Centers for Disease Control.
Taken together, the available evidence shows the updated vaccines produce good levels of antibodies in laboratory studies, in humans and mice when compared to previous vaccines and when looking at multiple emerging variants, including EG.5 (sometimes known as Eris). This variant is the one causing high numbers of cases around the world currently, including in Australia. It is very similar to the XBB version contained in the updated booster.
The updated vaccines should also cover BA.2.86 or Pirola, according to early results from clinical trials and the US Centers for Disease Control. This variant is responsible for a rapidly increasing proportion of cases, with case numbers growing in Australia.
It’s clear the virus is going to continue to evolve. So performance of these vaccines against new variants will continue to be closely monitored.
Are they safe?
The safety of the updated vaccines has also been shown to be similar to previous versions. Studies comparing them found no significant difference in terms of the adverse events reported.
Given the availability of the updated vaccines, some countries have removed their approval for earlier versions. This is because newer versions are a closer match to currently circulating strains, rather than any safety issue with the older vaccines.
What happens next?
The availability of updated vaccines is a welcome development, however this is not the end of the story. We need to make sure eligible people get vaccinated.
We also need to acknowledge that vaccination should form part of a comprehensive strategy to limit the impact of COVID from now on. That includes measures such as mask wearing, social distancing, focusing on ventilation and air quality, and to a lesser degree hand hygiene. Rapidly accessing antivirals if eligible is also still important, as is keeping away from others if you are infected.
Paul Griffin, Professor, Infectious Diseases and Microbiology, The University of Queensland
This article is republished from The Conversation under a Creative Commons licence. Read the original article.
When did you last have a COVID vaccine booster? Is it time you had another one? Let us know in the comments section below.
Also read: Can a COVID infection trigger dementia?


NO, just no.